Research
Cellular mechanisms and molecular control of tumor-host tissue interaction and invasion in medulloblastoma
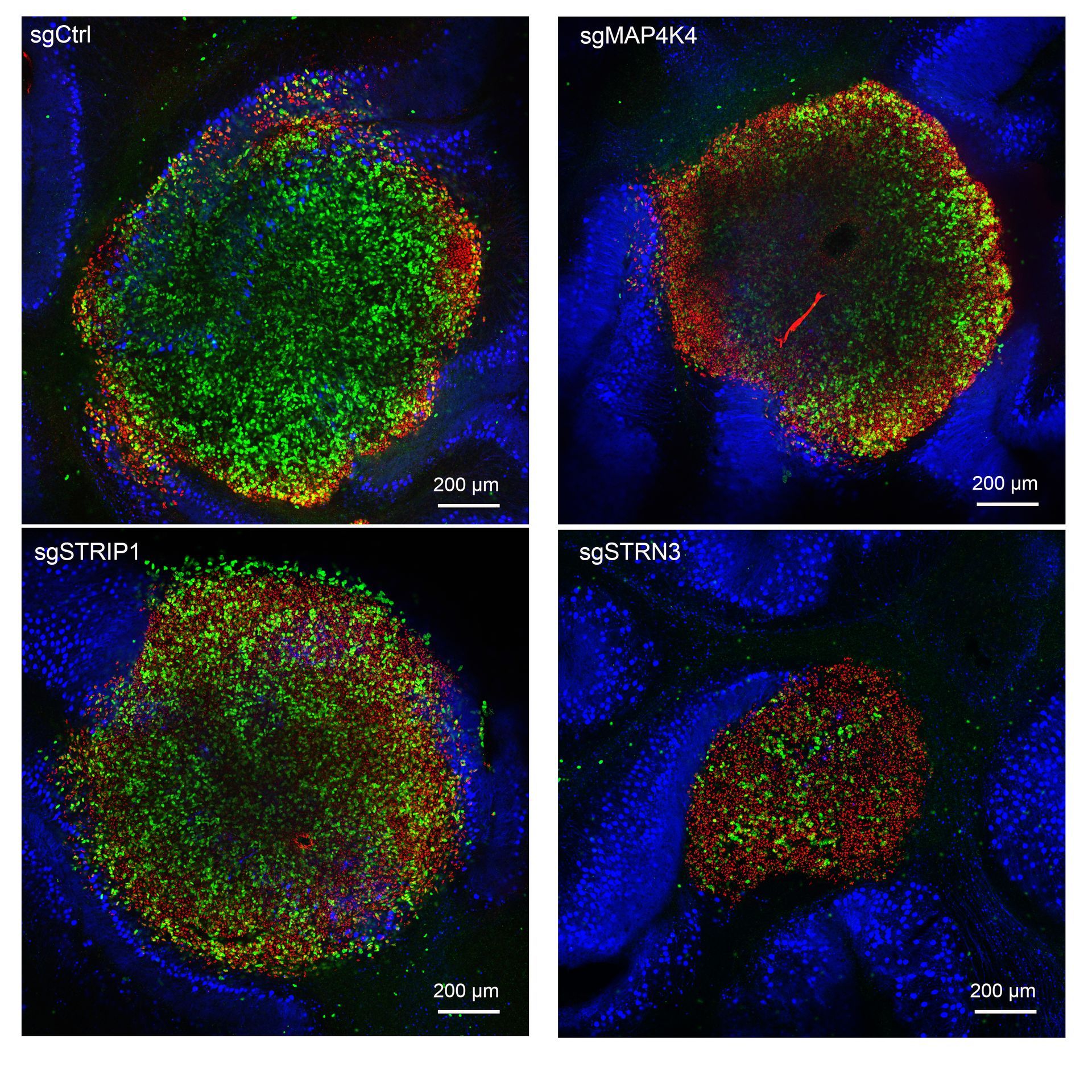
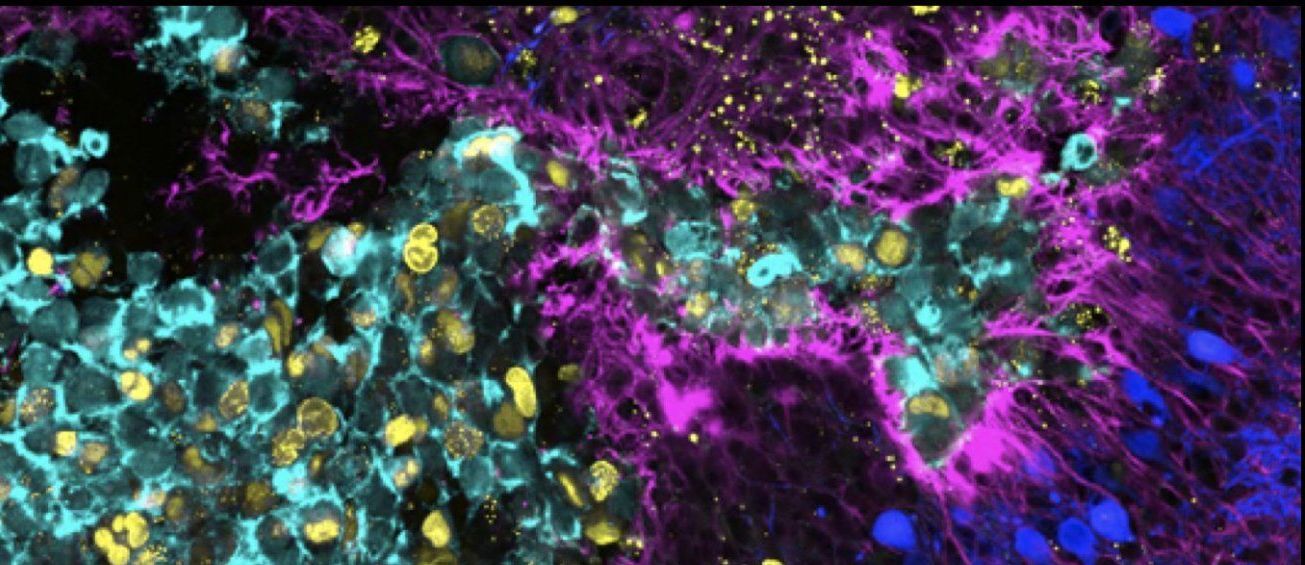
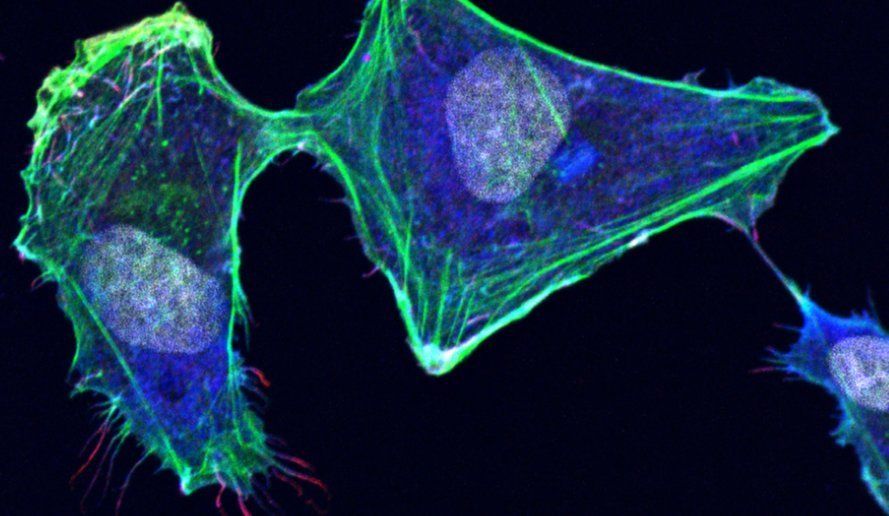
The biological principles underlying tumor-host tissue interactions in medulloblastoma (MB), as well as the key molecular regulators of cellular processes and morphological changes that drive tumor growth and tissue invasion, remain poorly understood. In particular, how MB tumor cells “communicate” with cells in the cerebellar microenvironment — and how the microenvironment responds — is not yet fully elucidated. Beyond soluble chemical factors, tumor cells release extracellular vesicles (EVs) and extend tunneling nanotubes (TNTs), both of which may facilitate the transfer of chemical signals between different cellular entities.
The primary objective of this study is to characterize the molecular mechanisms governing EV biogenesis and TNT formation in tumor-tumor and tumor-astrocyte crosstalk. Our secondary objective is to investigate the functional significance of MB-derived EVs and TNTs in tumor-host tissue interactions and tissue invasion.
We anticipate that this work will provide crucial mechanistic insights, laying the foundation for the rational design of mechanism-based therapies aimed at targeting tumor-induced deregulation of host tissue functions that promote tumor growth and invasion.
Drug discovery: RaSMID
SNF Sinergia Project:
Rational Small Molecule Inhibition of Dissemination (RaSMID) for pediatric brain tumors
The lack of effective therapeutics that precisely target the biological mechanisms driving tumor progression remains a obstacle to developing anti-metastatic treatments. Targeting protein-protein interactions (PPIs) is an emerging concept in drug discovery that holds promise for overcoming this challenge. When successfully implemented, it offers an innovative strategy to selectively inhibit or alter protein functions under pathological conditions.
Key prerequisites for discovering effective and safe small molecule PPI inhibitors include advanced computational (“in silico”) screening, robust functional validation technologies, and physiologically relevant model systems to assess the biological impact of compound activity and PPI disruption.
We apply this discovery approach to identify small bioactive molecules against medulloblastoma (MB), the most common malignant pediatric brain tumor. With the anticipated lead compounds targeting tumor dissemination and the establishment of novel methodologies for bioactive compound validation, we aim to contribute to ongoing efforts to develop effective and safe treatments for MB patients.
Our collaborators:
Prof. Gisbert Schneider, Computer-assisted Drug Design
Prof. Stephan Neuhauss, Neuhauss Group

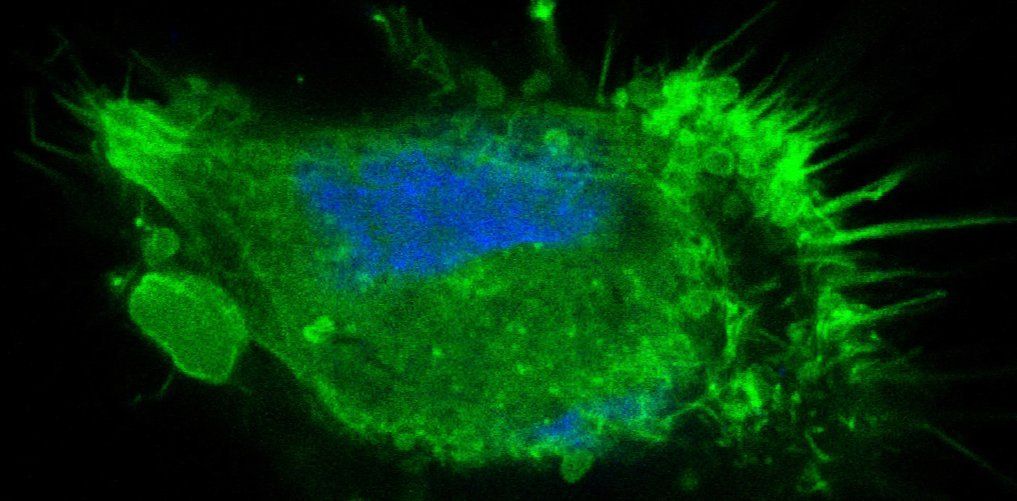
The coordinate remodeling of the actin cytoskeleton mediates the motility and invasiveness of cancer cells. It is the interplay of a plethora of signaling pathway components that orchestrates the underlying control of actin cytoskeleton remodeling. We combine pharmacological and genetic gînterference strategies with quantitative imaging tools to identify relevant signaling pathways that promote cell migration and tissue invasion and determine key molecular regulators in these pathways that act as therapy targets for existing drugs or novel compounds.

ONCOGENIC SIGNALING
Molecular signaling pathways control tumor cell behavior and shape the cellular response to anti-cancer therapies. Our research focuses on how pro-oncogenic signals in medulloblastoma cells are initiated, processed, and translated into a biological response. We specifically focus on kinase signaling pathways activated by receptor tyrosine kinases and adhesion receptors, which control actin dynamics, receptor turnover, and cell motility. Our objective is to modulate pro-oncogenic signaling such that the capability of the tumor cells to migrate and invade is repressed.
FGFR signaling promotes growth and tissue invasion in medulloblastoma. We rationally designed and develop a small molecule targeting strategy to block pro-invasive FGFR signaling. We furthermore explore whether novel combinations of existing drugs could enable efficient blockade of pro-metastatic cell functions.
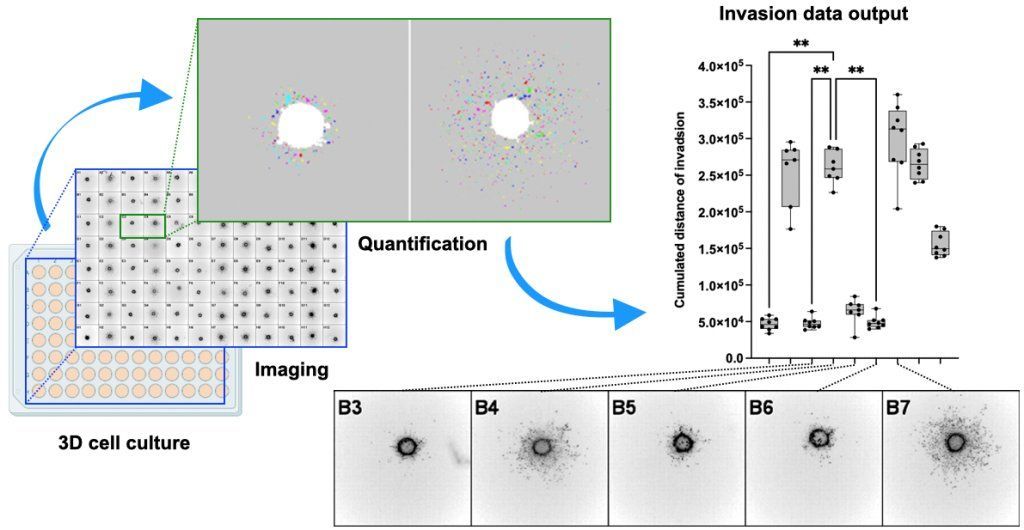
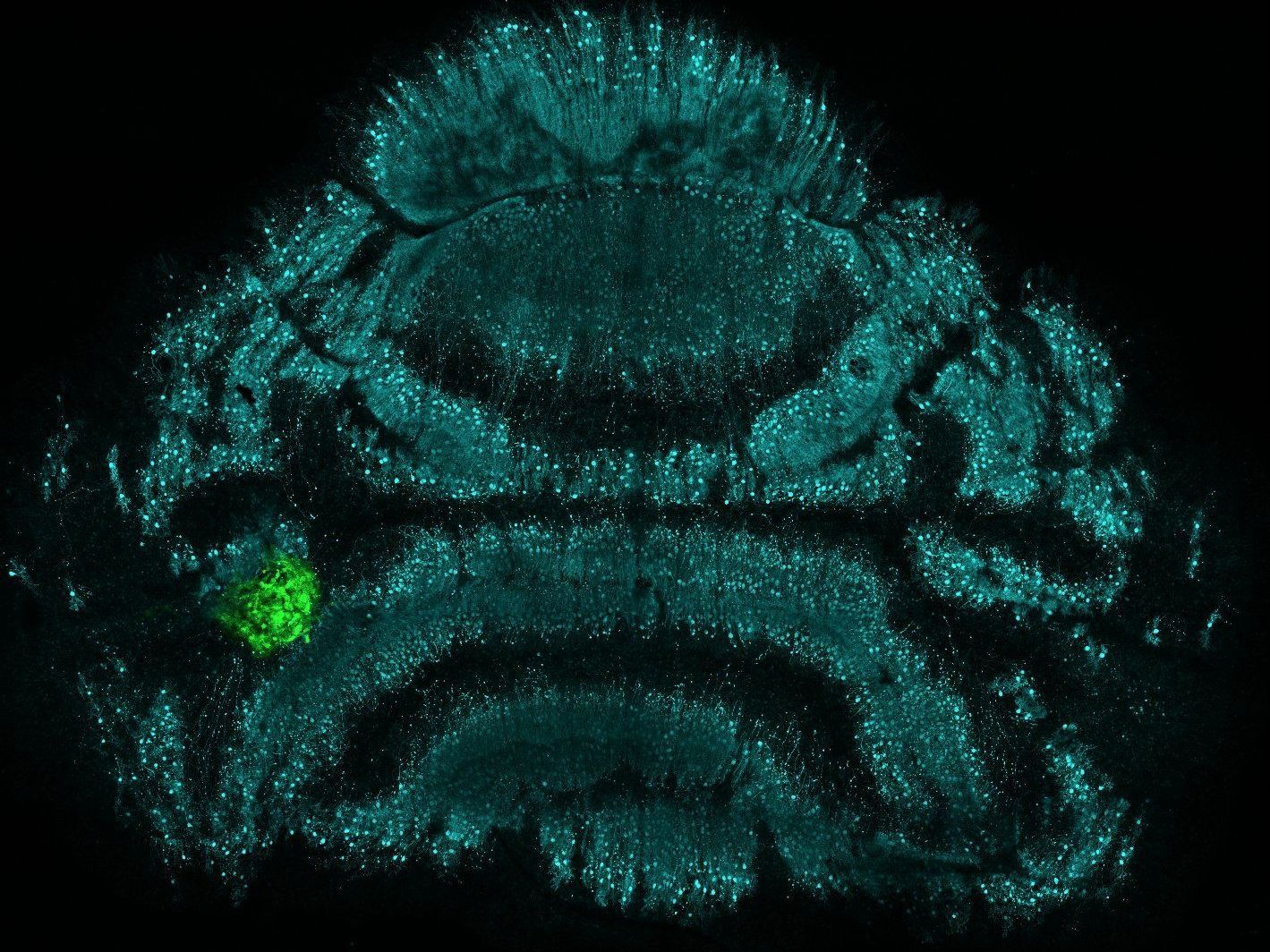
We develop a tissue model for pediatric brain tumor research that allows monitoring of cell behavior, tissue integrity and drug response in real time. Our established organotypic cerebellar slice culture system allows the end-point investigation of molecular mechanisms of tumor growth and tissue invasion and to test novel therapeutic approaches in a physiological environment ex vivo. Our objective is to develop this model further for real-time monitoring of biological responses in a physiologically relevant tissue environment.
Our funding sources: SNF, Swiss Cancer League/Krebsforschung Schweiz, Innosuisse, Kinderkrebsforschung Schweiz
USEFUL LINKS: